FDA's Fast-Track for Rexulti Raises Concerns
Por um escritor misterioso
Descrição
CMS efforts to reduce use of unnecessary antipsychotics in nursing homes may conflict with marketing efforts for the drug.

Fast-Track Drug Approval, Designed for Emergencies, Is Now Routine - WSJ
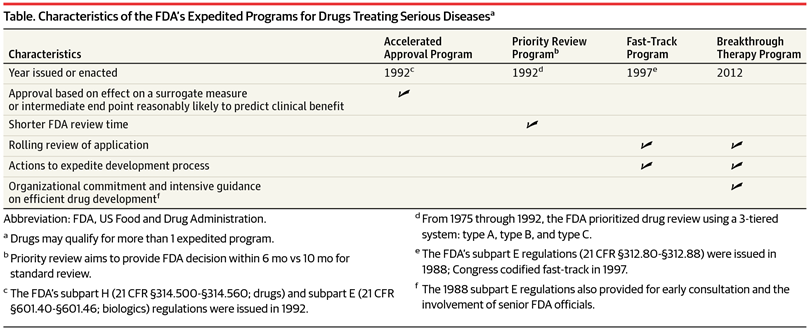
The Science Of A Biotech Valuation: How To Interpret The Value Of FDA Expedited Programs (NASDAQ:IBB)

FDA efficiency for approval process of COVID-19 therapeutics, Infectious Agents and Cancer

FDA-Approved Drugs to Treat Schizophrenia Journal of Psychosocial Nursing and Mental Health Services

FDA-Approved Drugs to Treat Schizophrenia Journal of Psychosocial Nursing and Mental Health Services

Controversy Surrounds Brexpiprazole's FDA Approval, a New Alzheimer's Agitation Drug

FDA-Approved Drugs to Treat Schizophrenia Journal of Psychosocial Nursing and Mental Health Services

Amgen Triumphs in $27.8 Billion Horizon Buyout Battle
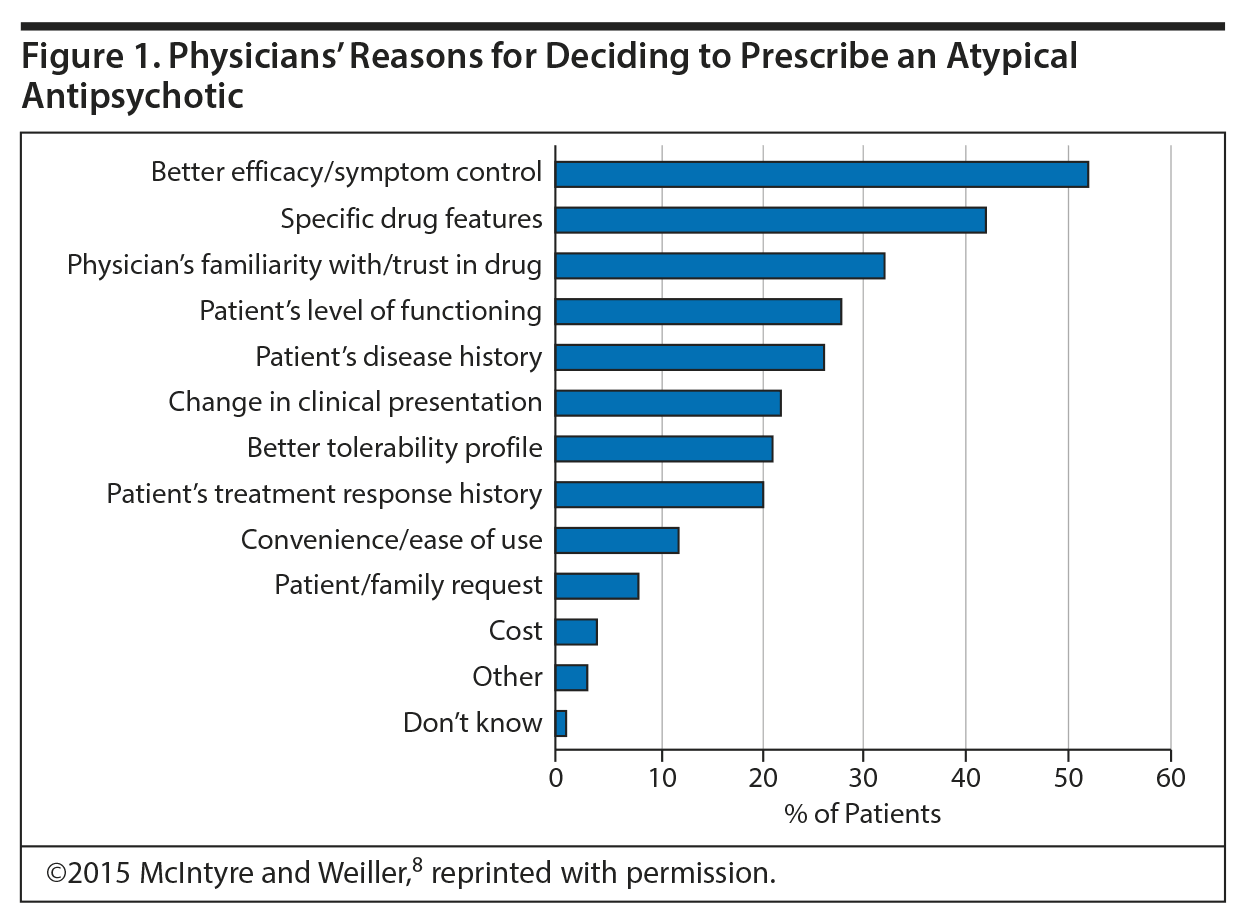
Activating and Sedating Properties of Medications Used for the Treatment of Major Depressive Disorder and Their Effect on Patient Functioning
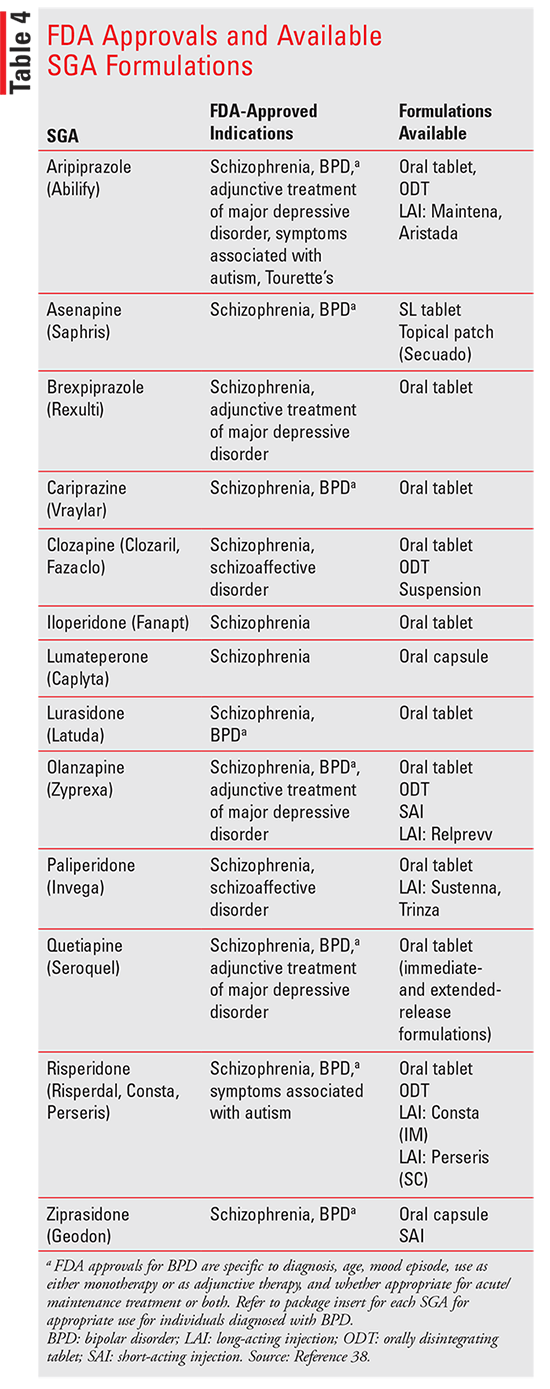
Lesson: Assessing the Current Antipsychotics Landscape

FDA approves first drug meant to ease Alzheimer's-linked agitation

REXULTI® (brexpiprazole)
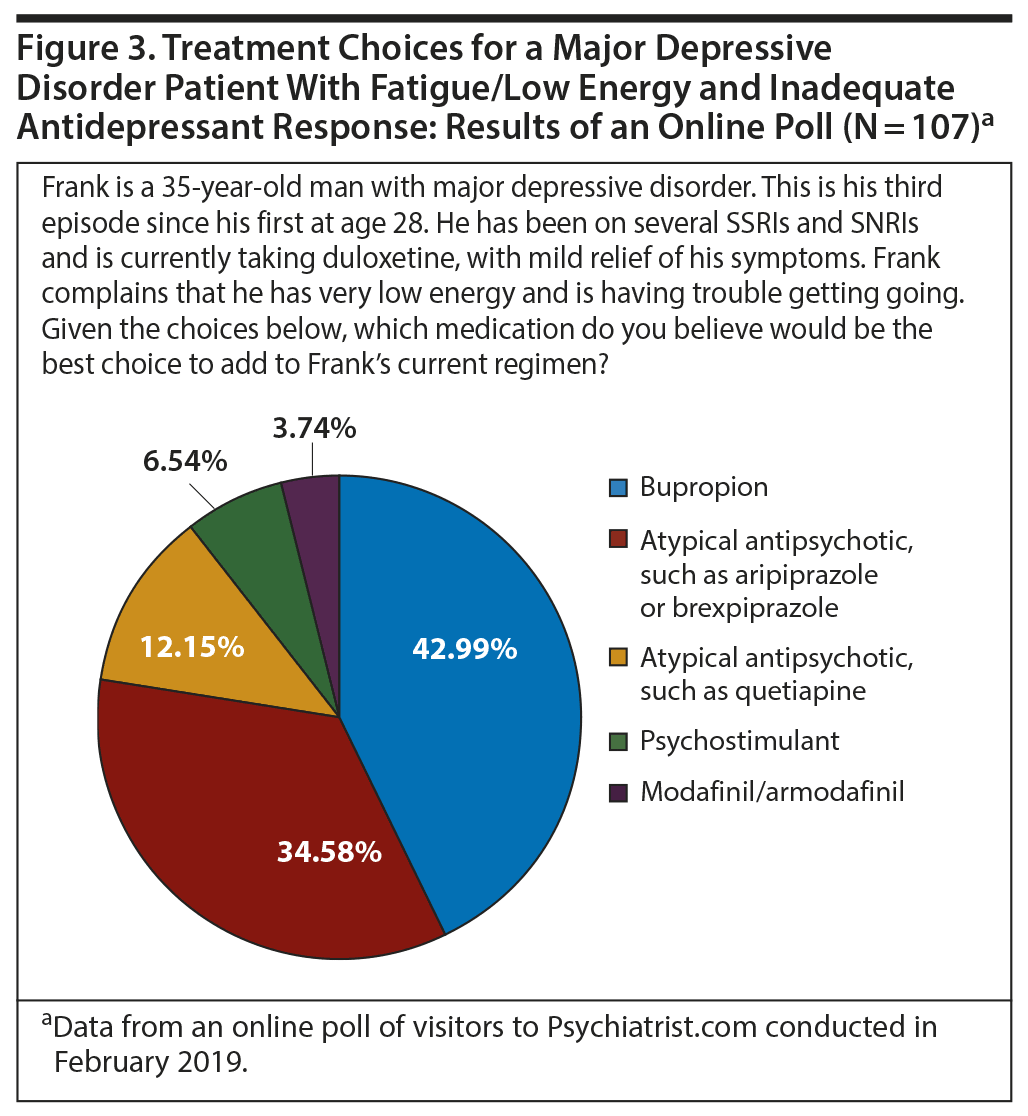
Activating and Sedating Properties of Medications Used for the Treatment of Major Depressive Disorder and Their Effect on Patient Functioning
de
por adulto (o preço varia de acordo com o tamanho do grupo)






